BiQ Journal: March 15, 2025 (PRLD, FHTX)
I come from a science-heavy background but pivoted to business instead of continuing on to medical school (I didn't want all the debt!) Over my career, I have launched three startups, two of which I helped guide through multiple private equity funding rounds. It's been quite a journey, and I'm forever grateful for the amazing opportunities life has gifted me.
After exiting my last startup, I was in the enviable (yet often frustrating) position of trying to figure out what I wanted to do next. Another startup? Go back and finish medical school? I honestly had no idea, but I did know one thing: at this point in my career, I wanted to do something I enjoy–and I had no desire to get back into the startup race. Startups can be a fun adventure, but you blink, and the next thing you know, ten years of your life have gone by. Those of you who have ever launched a startup know what I mean.
So, I had to think about what I genuinely enjoyed enough to keep me interested and occupied. Some people love Diablo. Others love pottery or golf. I happen to love biotech investing (plus I'm terrible at all three of the other options). I've been investing in biotech for a long time but only decided to launch Biotech iQ as a labor of love about a year ago for a small group of investors who had been following me for years and wanted an easier way to keep track of my ideas. A little over two months ago, I decided to launch BiQ publicly. And that brings us to where we are today.
I still do some consulting work to pay the bills, but leading Biotech iQ allows me to spend the rest of my time happily researching promising biotech and medtech companies. Most of these never make it into the BiQ Active Portfolio, but every now and then, I stumble onto a gem that meets my criteria for Biotech iQ. But, as they say, it's the journey that matters, not the destination.
Short story long, this brings me to this very first entry in the BiQ Journal. I research a lot of companies, some of them promising, some of them perhaps less so. Even the promising ones, however, are usually not sufficiently de-risked for me to invest in. Still, they are nonetheless fascinating–or at least fascinating to me, and perhaps to others. That's the idea behind the BiQ Journal. Most of the companies discussed here do not meet the stringent investing criteria I use for BiQ; however, I still think many of these stories are worth following and might be worth sharing with BiQ members.
Prelude Therapeutics (PRLD) and Foghorn Therapeutics (FHTX) are developing selective protein degraders to treat hard-to-treat cancers. More specifically, today I'll focus on their SMARCA2 degraders for cancers exhibiting SMARCA4 mutations.
SMARCA2 and SMARCA4 are proteins that are involved with the regulation of gene expression through chromatin remodeling. Without these proteins, cells would die due to an inability to express critical genes and repair or replicate DNA. In the case of SMARCA4-mutated cancers, cancer cells must rely solely on SMARCA2 to allow for chromatin remodeling. Degrading SMARCA2 in cells with SMARCA4 mutations, therefore, leads to cell death. This concept is known as synthetic lethality.
SMARCA4 mutations are observed in ~5% of all solid tumors and up to ~10% of all NSCLC patients. The standard of care for NSCLC with SMARCA4 mutations is chemotherapy and is associated with dismal survival outcomes, exhibiting a mPFS of ~2.7 months and ORR of ~22% in a first-line setting. The prognosis for later lines of treatment can be presumed to be even worse. This makes SMARCA4-mutant NSCLC an area of very high unmet need.
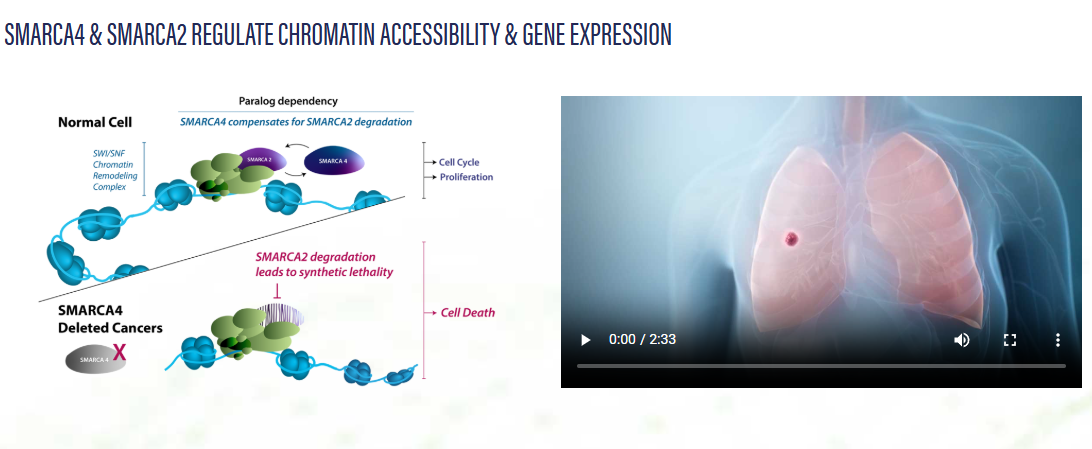
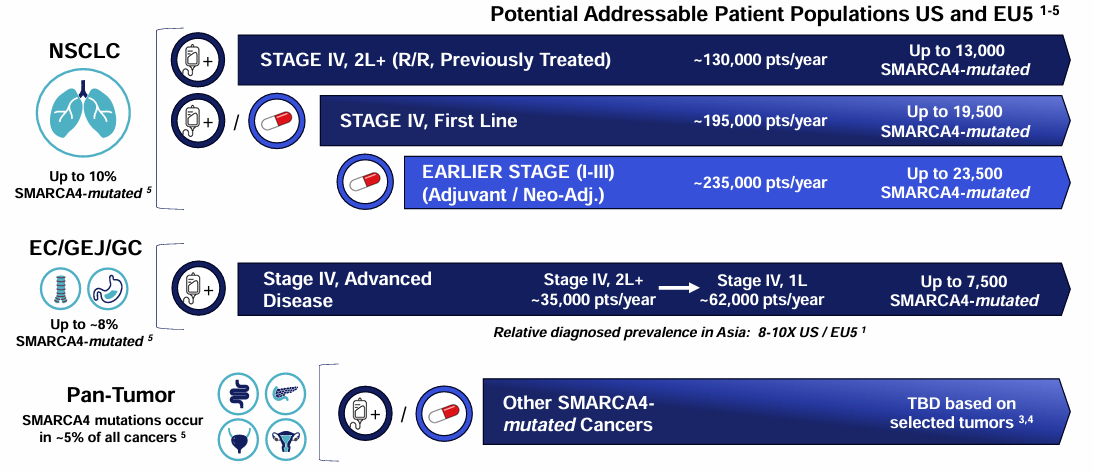
This isn't a new concept; however, past efforts have been unable to target SMARCA2 with sufficient selectivity to avoid systemic toxicity issues.
Prelude Therapeutics is advancing PRT3789 and PRT7732 through clinical trials to address this unmet need. PRT3789 is delivered via IV infusion and exhibits 1000x more selectivity for SMARCA2 vs. SMARCA4. PRT7732 is delivered orally and exhibits 3000x selectivity. Presumably due to this high level of selectivity, tolerability in early clinical models has been favorable both in monotherapy and in combination with Docetaxel:
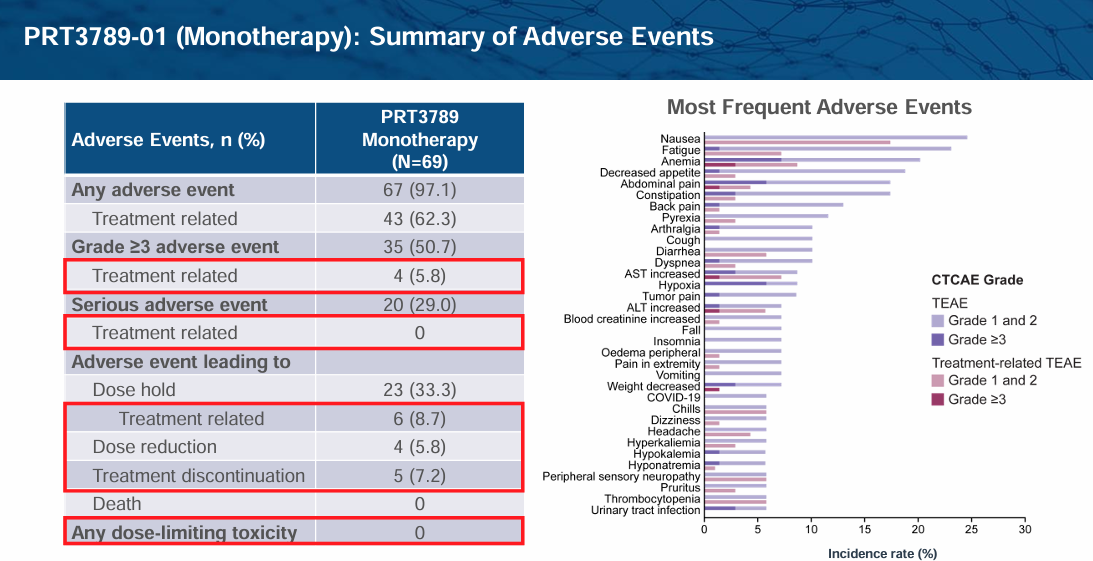
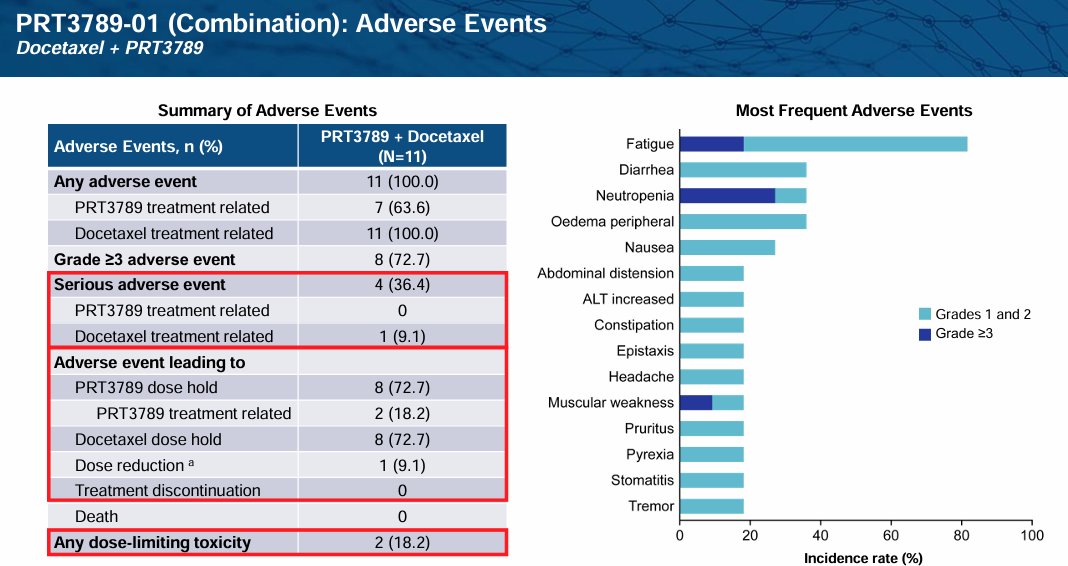
PRT3789 in monotherapy has also demonstrated early signs of efficacy, especially in higher-dose cohorts:
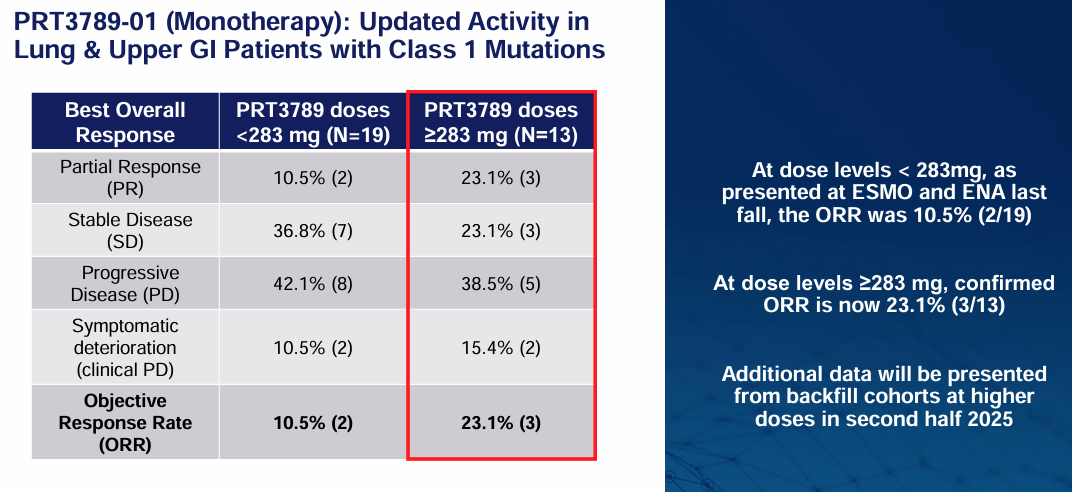
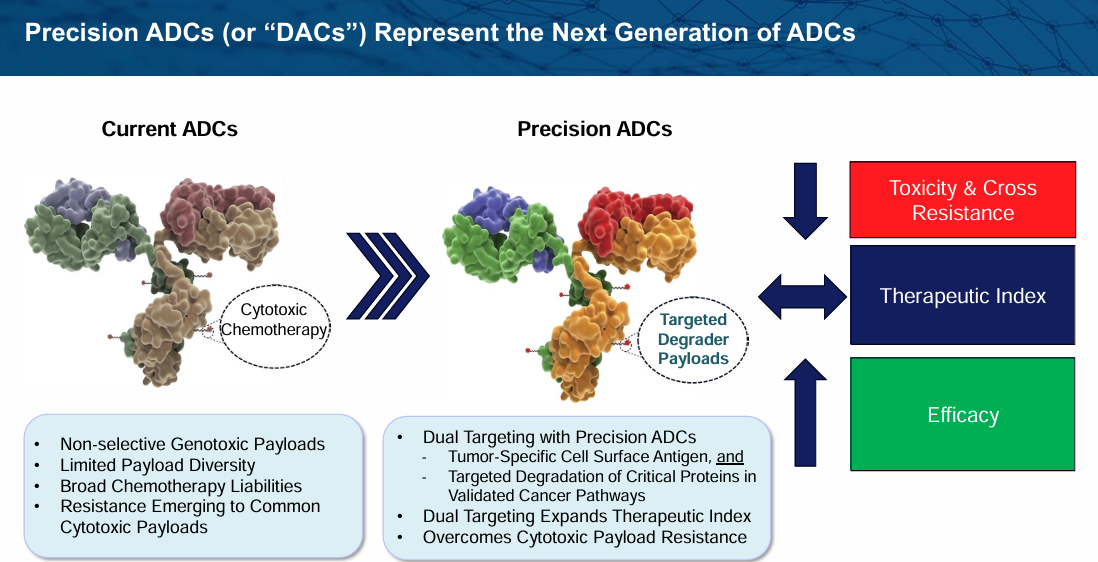
What I find even more interesting is Prelude's Degrader Antibody Conjugate, or DAC, which features a tumor-antigen targeted antibody combined with a highly selective degrader payload. This allows for a dual-targeting mechanism where both the surface antigen and the specific protein are selectively targeted, potentially leading to even better tolerability and a higher therapeutic index. Prelude plans to present additional data on their SMARCA2 degrader programs in the second half of 2025.
Foghorn Therapeutics is partnered with Eli Lilly and is developing both a selective SMARCA2 inhibitor (FHD-909) and a SMARCA2 degrader, which is still in preclinical development. Personally, I'm more interested in the degrader than the inhibitor. In this situation, degraders may have an advantage over inhibitors because it takes longer for SMARCA2 to recover after being degraded vs. inhibited, which should allow for a more favorable dosing schedule. I assume this is why Foghorn is developing its own SMARCA2 degrader despite already having an inhibitor in the clinic. Unfortunately, no clinical data is available yet for FHD-909; however, I believe the company plans to present early data in April at AACR.
It's still early days, and neither of these companies is sufficiently de-risked enough for the BiQ Active Portfolio; however, I think both are interesting, and I look forward to watching further developments in this space.
I would love to hear your feedback! If you have any questions or comments (or corrections!) to share regarding this article, please click the "Comments" icon below to go to the online version of this article, scroll to the bottom of the page, and enter your question or comment in the Member Discussion area.
The BiQ Active Portfolio consists of my top biotech and medtech ideas and is updated regularly for BiQ Premium members. You can go to the Performance page to view my past and current performance. If you would like to try out a BiQ Premium membership, please click the blue Account or Subscribe button on your screen to sign up for a risk-free 7-day trial membership. Or, if you're ready to join BiQ, click here for information on how to get a 20% discount on an annual subscription for life.
Biotech iQ is 100% subscriber supported. If you find this information helpful, please spread the word. You can also follow me on X @ Biotech iQ (_Biotech_iQ).
Biotech iQ is not an investment professional, and nothing on this page or this website should be considered investment advice. Please consult with a licensed investment professional as necessary. Past performance is not indicative of future results.
Member discussion