BiQ: PRGN-2012 vs INO-3107: Two Strong Contenders (PGEN, INO)
As BiQ Premium members know, Precigen (PGEN) is a clinical-stage biotech I have been following for some time at Biotech iQ. The company is developing very intriguing technology with its AdenoVerse Gene Therapy and UltraCAR-T platforms. My previous articles and updates on PGEN can be found here: 01/16/25, 02/28/25, 03/08/25.
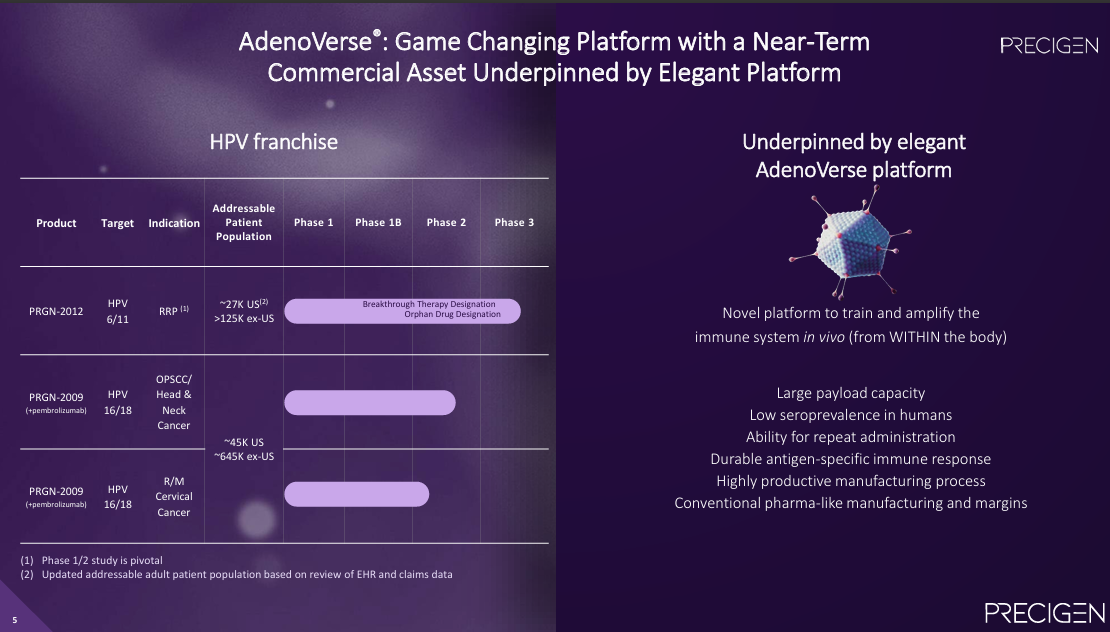
As a quick refresher, AdenoVerse is an off-the-shelf gene therapy platform using PGEN's proprietary gorrilla adenovectors which feature a large payload capacity and the ability to be re-dosed without generating neutralizing antibodies. AdenoVerse has the potential to target a wide variety of genetic diseases, but the company's initial focus is on HPV-related cancers.
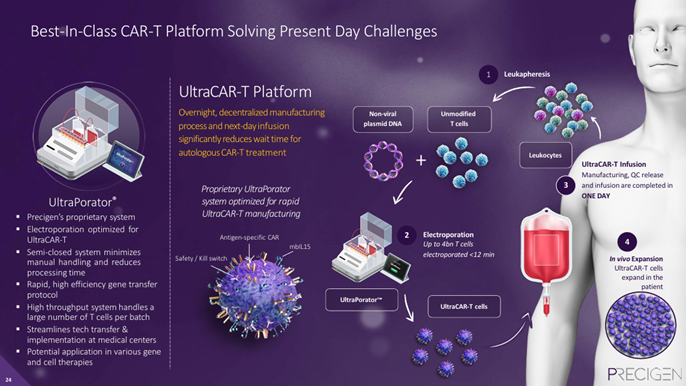
UltraCAR-T has the potential to provide overnight, decentralized manufacturing of autologous CAR-T therapies that feature in-vivo T-cell expansion, a kill switch, and provide intrinsic CPI blockade--eliminating the need to combine with CPIs.
Precigen recently filed a BLA under the accelerated approval pathway for PRGN-2012 for the treatment of Recurrent Respiratory Papillomatosis (RRP) and has a PDUFA target date of 08/27/25. Thanks to BiQ Premium member @DamianMart, however, we recently discovered that competitor Inovio Pharmaceuticals (INO), another genetic medicines company, is preparing its own BLA filing for INO-3107 to treat RRP which it plans to submit in 2025 H2, also under the accelerated approval pathway.
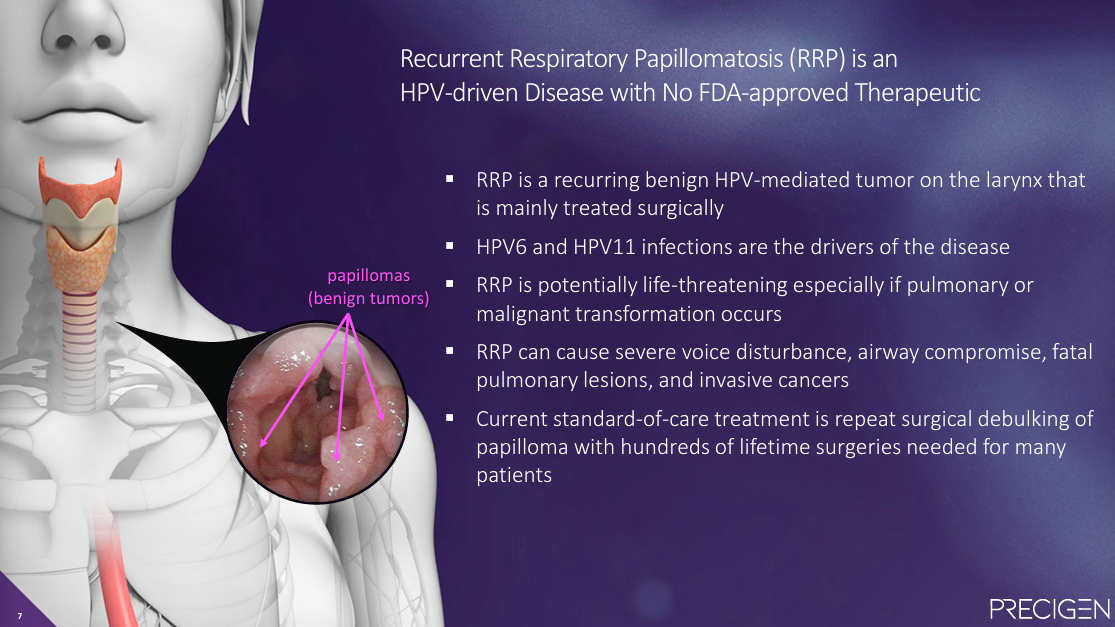
RRP is driven by HPV6 and HPV11 infections, which cause recurring benign tumors on the larynx. There is currently no targeted treatment, and standard-of-care consists of repeated surgical debulking of papilloma, with some patients needing hundreds of surgeries over their lifetime. These repeated surgeries often lead to permanent damage to patients' larynx and vocal cords and, in some cases, can also lead to pulmonary issues and malignancies, which can be fatal.
Estimates of disease prevalence vary. In its literature, Inovio estimates 14K prevalent cases in the US, while Precigen estimates 27K in the US and 125K ex-US. Either way, RRP represents a significant unmet need.
Precigen and Inovio each have very interesting platforms with significant potential, and both are advancing late-stage candidates for the treatment of RRP. In the paragraphs below, we'll focus on PRGN-2012 and INO-3107 to see which company, if any, may hold an advantage in this potentially lucrative area of high unmet need.